Today, the US Food and Drug Administration (FDA) released draft guidance on plant-based milk labeling. According to the agency, the draft guidance, when finalized, is non-binding and will represent the current thinking of the FDA on the topic of naming and voluntary nutrient labeling of plant-based milks.
In summary, the agency now says plant-based milk alternatives can be called “milk”, but recommends such products to identify certain nutritional differences from cow’s milk on their front-of-package labeling.
“Imposing arbitrary regulatory hurdles that disadvantage the plant-based dairy industry is the last thing the FDA should be doing”
In the guidance document, the FDA notes it has not established compositional requirements for plant-based milk alternatives, and that such products comprise a wide diversity of ingredients and methods of production.
Consumers not confused
The agency also acknowledges that, as evidenced by numerous consumer studies and its own focus groups, consumers are not confused by the use of the term “milk” on non-dairy products. “Focus groups commissioned and conducted by FDA suggested that ‘milk’ is strongly rooted in consumers’ vocabulary when describing and talking about plant-based milk alternatives,” the draft guidance states. “The focus groups indicated that most participants were not confused about plant-based milk alternatives containing milk and refer to plant-based milk alternatives as ‘milk.’”

It adds, “Participants further indicated that they feel familiar and comfortable with the term “milk” when describing plant-based milk alternatives and they preferred to use the term when given a choice of names for plant-based milk alternatives (e.g., “milk,” “beverage,” “drink,” etc.).”
Nutritional differences
However, when assessing the nutritional distinctions between plant-based and cow’s milk, the agency asserts consumers do not fully understand important nutritional differences between the products.
“In general, research suggests that many consumers lack an accurate understanding about the specific nutrients in plant-based milk alternatives,” the guidance reads. ”The research also suggests that a majority of consumers who purchase plant-based milk alternatives state they do so because they believe the products are healthier than milk.”

New recommendations
It continues, “[F]ocus groups conducted by FDA with consumers of plant-based milk alternatives, frequent mentions were made that plant-based milk alternatives may be healthier than milk because they are lower in fat and cholesterol, and do not contain animal ingredients. Further, a survey reported that 53 percent of its respondents believe that plant-based milk alternatives labeled with the term “milk’ in their name have a nutritional content similar to milk.”
Taking issue with these potential discrepancies, and to ensure that plant-based milk labels “help enable consumers to quickly ascertain the attributes of products they are purchasing”, the agency has set forth new labeling recommendations for front-of-pack labels.
Illustrated examples are as follows:


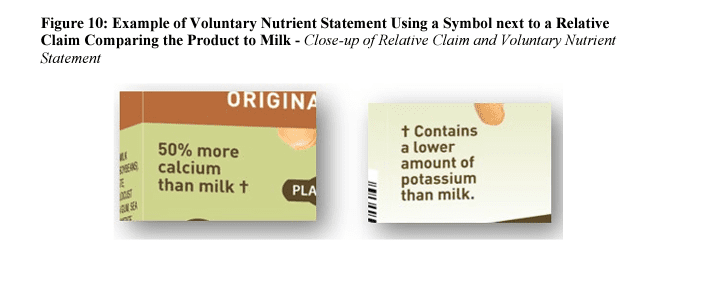
Industry reaction
Though the draft guidance is nonbinding and does not carry the force of law, industry groups, including The Good Food Institute (GFI), call the recommendations an “arbitrary” burden that unfairly disadvantages plant-based milk producers.
“GFI supports commonsense labels that use terms consumers understand and themselves use,” says GFI senior regulatory attorney Madeline Cohen in a reaction statement. “The government’s role is to ensure a level playing field. FDA should not impose de facto labeling requirements on plant-based milks while giving cow’s milk a free pass.”

She continues, “Despite acknowledging consumers’ familiarity with plant-based milks, the guidance misguidedly admonishes companies to make a direct comparison between their plant-based milks and cow’s milk. The guidance is premised on the idea that consumers are somehow confused by plant-based milks’ nutrition, despite the fact that FDA already requires key nutrients to be included on the Nutrition Facts panel.”
Double standards?
Cohen also points out the FDA does not require cow’s milk producers to identify nutritional differences between its own products, e.g. skim milk and reduced-fat chocolate milk:
“The guidance compares plant-based milks to one standardized milk product even though FDA has never required any particular nutrient content for cow’s milk,” states Cohen. “Milks such as unfortified skim milk and 2% reduced-fat chocolate milk have significant nutritional differences from whole cow’s milk, yet these products are not required to note them on front-of-pack labels. Moreover, the guidance urges disclosures about nutrients that are not under-consumed by Americans, such as magnesium, and even nutrients that are overconsumed by some groups, such as protein.”

According to Cohen, such requirements could have serious implications for US environmental policy. “The guidance undercuts the administration’s climate goals. If the US is serious about meeting its climate commitments, imposing arbitrary regulatory hurdles that disadvantage the plant-based dairy industry is the last thing the FDA should be doing.”
The FDA’s draft guidance can be viewed here.





